EU Health - #HealthUnion on X: "Adapted vaccines target different variants & can help maintain optimal protection as the virus evolves Today, we've authorised Moderna's BA.4/BA.5 adapted booster vaccine, following @EMA_News positive

News - The European Commission and the Committee for Medicinal Products for Human Use at the EMA Voted Positively for mRNA Booster Vaccinations from BioNTech/Pfizer and Moderna Adapted to the Omicron Virus
ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines | CDE Almería - Centro de Documentación Europea - Universidad de Almería
Third COVID-19 dose approved by EMA 28 days after second vaccination - Hospital Pharmacy EuropeHospital Pharmacy Europe
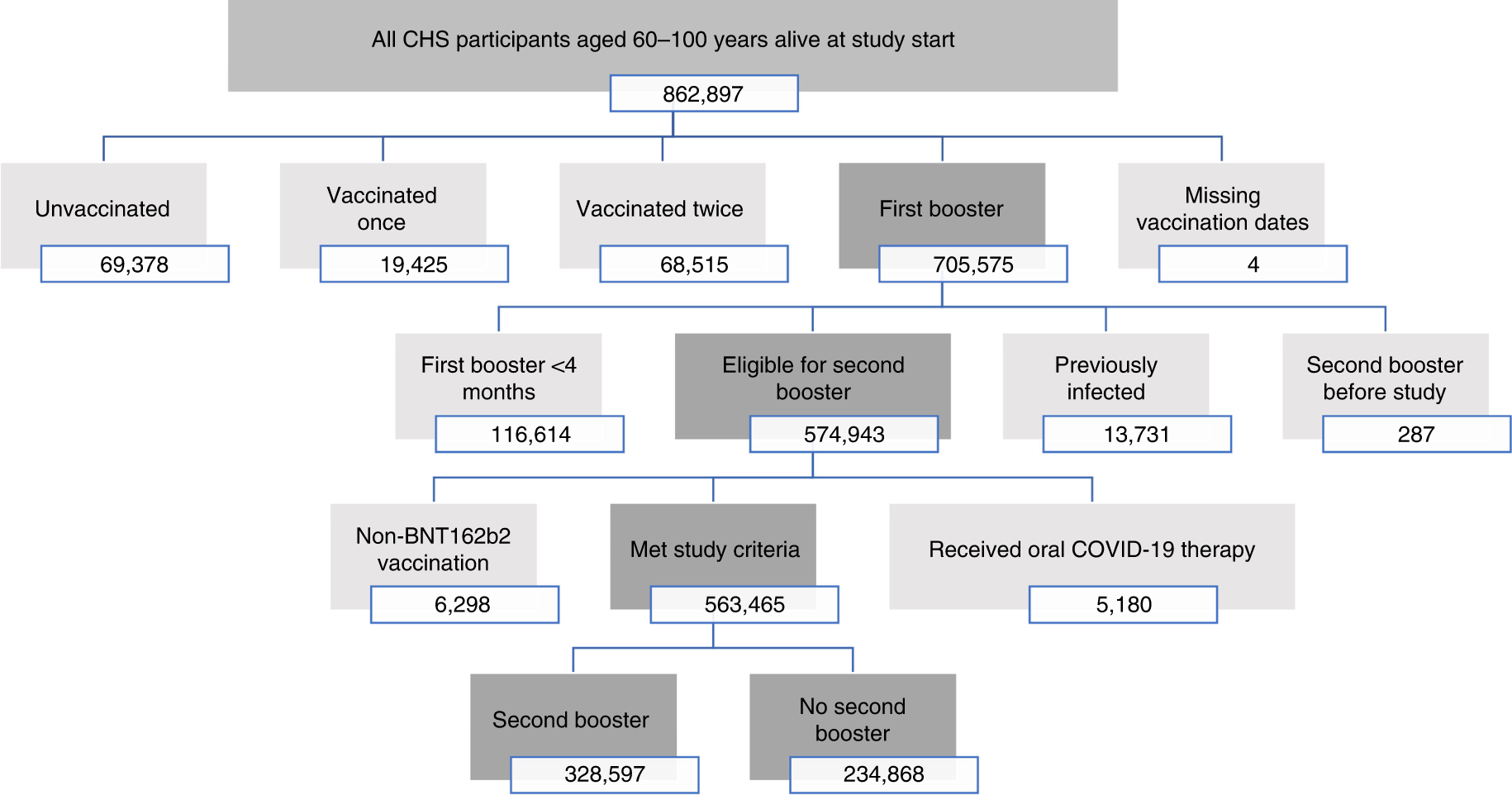
Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years | Nature Medicine

European Medicines Agency Recommends Second COVID Booster For People Over 60 - As WHO Ponders Status Of COVID Emergency - Health Policy Watch

EMA evaluating data on booster dose of COVID-19 Vaccine Janssen | CDE Almería - Centro de Documentación Europea - Universidad de Almería